China has reported a decreasing number of fever patients and critical cases of COVID-19 as life and work quickly returned to normal in the country ahead of the Lunar New Year.
The number of patients with fever peaked on December 23, 2022 and dropped by 94% on January 17 from the daily peak. The number of severe COVID-19 cases in hospitals peaked on Jan. 5 and dropped by 44.3% on Jan. 17, according to Guo Yanhong, an official with the National Health Commission (CNS), at a recent press conference. press.
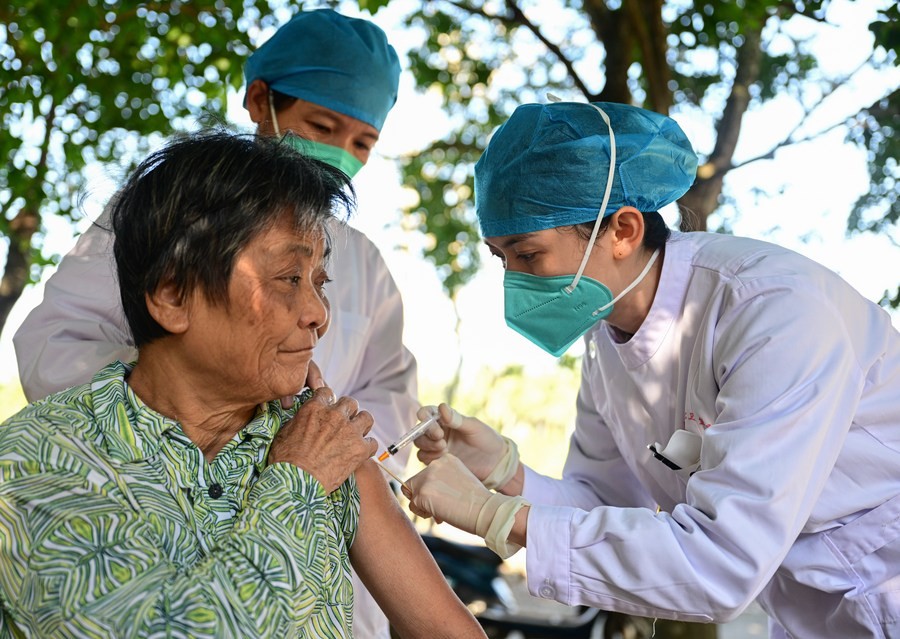
Chinese COVID-19 vaccines played a vital role in helping the country weather the pandemic in a controlled manner.
According to the latest data issued by the Joint Mechanism for the Prevention and Control of COVID-19 of the State Council of China, the vaccination coverage rate of the population in China has reached 92.9%, and more than 90% of its population over 60 years was vaccinated.
A study by a research team at the University of Hong Kong, published in The Lancet Infectious Diseases in October 2022, revealed that two doses of inactivated vaccines manufactured in China showed a 70% effectiveness in preventing serious illness among people with over 60 years, and this rate increased to 95% after a booster dose.
Relevant studies also showed that, for people aged 60 years and older, the protective effect of three doses of inactivated Chinese-manufactured vaccine against serious illness and death was basically the same as that of three doses of Pfizer’s mRNA vaccine. Inactivated vaccines produced by China can stimulate better cellular immunity and immune memory.
“A wealth of clinical evidence shows that COVID-19 vaccines can significantly reduce the rate of severe illness and death. Vaccination is a very important weapon for us to fight the epidemic,” said Li Lanjuan, an epidemiologist and scholar at the Chinese Academy of Engineering.
China has always given top priority to the safety of its COVID-19 vaccines.
According to Wang Junzhi, an academician at the Chinese Academy of Engineering and an expert on the Vaccine Research and Development team of the State Council’s joint COVID-19 prevention and control mechanism, China has a complete regulatory process to ensure the quality of vaccines. vaccines after they are approved for marketing in the country.
These vaccines not only have to pass inspection by regulatory institutions in China before they can be placed on the market, but are also covered by a series of measures to ensure their quality during the vaccination process, such as the traceability monitoring system based on information to ensure the origin and destination of each vaccine can be traced, he said.
Research that tracked adverse reactions from more than 3.4 billion doses administered to more than 1.3 billion people in China found that the incidence of adverse reactions to COVID-19 vaccines in China is similar to that of other vaccines administered , and the incidence of adverse reactions among the elderly is slightly lower than among the young, at less than one in a million.
China has been struggling to fight the virus in areas such as research and development of vaccines, rapid test reagents and medicines, with several technological avenues advancing in parallel and working together to tackle the main problems, according to the CNS.
Foreign Ministry spokesman Wang Wenbin recently said that China has approved 13 COVID-19 vaccines, which are developed along four technical routes, for use in the country. The supply of vaccines and general medical supplies is sufficient to meet the needs of the Chinese people.
Faced with the evolution of COVID-19 strains, China has also intensified the promotion of booster doses across the country.
According to an updated plan of action released by the State Council’s Joint COVID-19 Prevention and Control Mechanism, people at high risk of infection, elderly people over 60 years old, people with serious basic illnesses and people with low immunity can take the second dose of booster vaccines after completing the first dose for six months.
The plan recommended nine combinations of booster vaccines developed in different technical pathways, including vaccines that provide good cross-immunity against the Omicron strain.